Plastics comprise a wide range of synthetic or semi-synthetic organic compounds, typically high molecular weight polymers. While there is no universal definition of the term microplastics, here I consider them to be fragments of plastic less than 1mm in size. There are two main classes of microplastics. Primary microplastics are any plastic fragments or particles already 1mm in size or less before entering the environment, including microfibers from clothing, microbeads, and plastic pellets known as nurdles. Secondary microplastics are microplastic particles created from the degradation of larger plastic products through natural weathering processes once they enter the environment.
So why bother looking at microplastics? Although there is now a growing literature on microplastics in aquatic environments, less is known about terrestrial ecosystems (Rillig, M. & Lehmann, A. (2020) Microplastic in terrestrial ecosystems. Science, 368 (6498), 1430-1431), and specifically the effects of microplastics on soil mesofauna, even though there is evidence that they may negatively affect soil fauna (Lin, D. et al. (2020) Microplastics negatively affect soil fauna but stimulate microbial activity: insights from a field-based microplastic addition experiment. Proceedings of the Royal Society B, 287, 1934, 20201268).
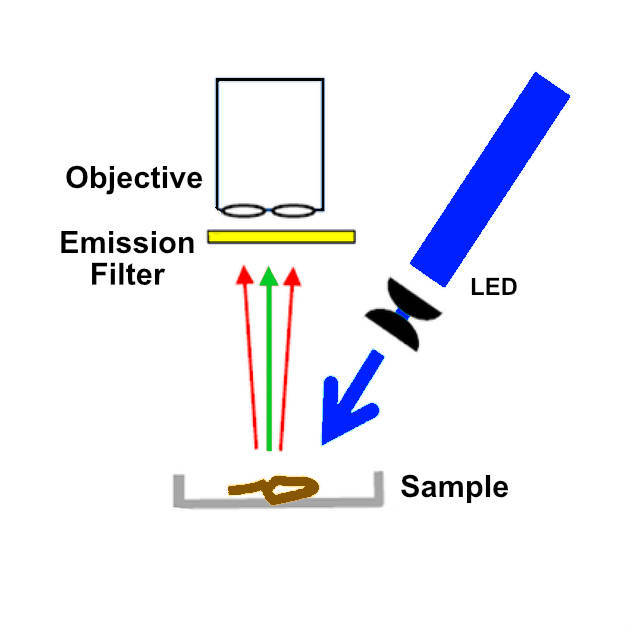
A few months ago a came across a paper with a simplified methodology for examining microplastic contamination in biological specimens (Labbe et al (2020) Inexpensive Adaptations of Basic Microscopes for the Identification of Microplastic Contamination. J. Chem. Educ. 97, 11, 4026–4032). This paper uses Nile Red to visualise microplastic particles, a lipophilic stain most frequently used to localize and quantitate lipids, particularly lipid droplets within cells (Greenspan P, et al. (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. The Journal of Cell Biology 100 (3), 965-973). Polar lipids (i.e. phospholipids) which are mostly present in membranes, are stained in red whereas neutral lipids (cholesterol and triglycerides), present in lipid droplets, are stained in yellow. Nile Red is almost nonfluorescent in water and other polar solvents but undergoes fluorescence enhancement and large absorption and emission blue shifts in nonpolar environments (excitation/emission maxima ~552/636nm in methanol). In the paper, a 1mg/ml solution of Nile Red in methanol is used to stain the particles. After staining fluorescence is visualised by illumination of the material with a blue light source and a yellow emission filter. As in the paper, I investigated using a blue laser to get maximum fluorescence but abandoned this in favour of a blue LED flashlight for safety reasons.
Helpfully, in the European Union most plastics intended to be recycled are labelled with a numbered code:
1 - PET (Polyethylene Terephthalate)
2 - HDPE (High Density Polyethylene)
3 - PVC (Polyvinyl Chloride)
4 - LDPE (Low-Density Polyethylene)
5 - PP (Polypropylene)
6 - PS (Polystyrene)
7 - Other Non Recyclable Plastic
With everything in place, I began by testing on some known (from the recycling codes) plastics. Hydrophobic plastics such as polyethylene show yellow-green emission in the presence of Nile Red, while more hydrophilic plastics show red emission. Native cellulose fails to fluoresce but appears blue due to breakthrough of scattered light. A fragment of clear plastic food packaging labelled 1: R-PET (Recycled polyethylene terephthalate) showed only faint red fluorescence on some of the abraded surfaces:
A piece of white food packaging labelled 7: Other Non Recyclable Plastic - gave a brighter yellow emission, again mostly from abraded surfaces:
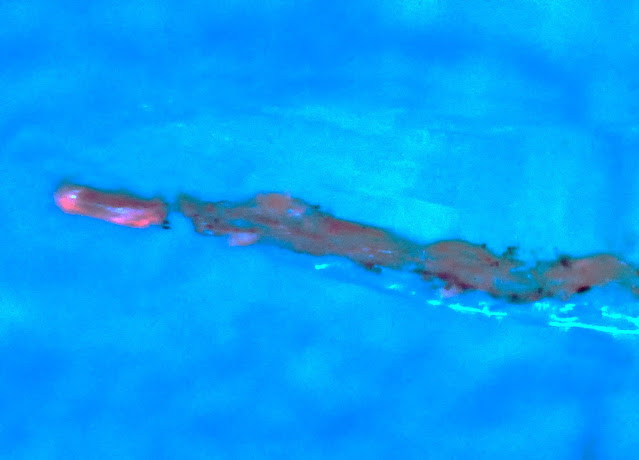
Dicyrtomina saundersi also gave a faint pattern of surface fluorescence, although the claws showed strong red fluorescence:
I then cleared some specimens of these two species with 10% NaOH and stained them after clearing. Very little fluorescence was visible in the D. saundersi but the O. cincta showed a bright pattern of surface fluorescence:
In none of the six specimens I examined did I detect any microplastic particles. However, these samples came from a relatively pristine environment (PAWS - planted ancient woodland sites), or at least, as pristine as it is possible to get in Leicestershire. Next I plan to look at samples from more urban environments where incidence of microplastic contamination is likely to be higher.




No comments:
Post a Comment
Comments welcome, I will respond as soon as I can.